Materials design of perovskite solid solutions for thermochemical applications
Publication Type
Date Published
Authors
DOI
Abstract
Perovskites are excellent candidate materials as oxygen carriers in thermochemical processes. Due to their versatile composition, it is possible to fine-tune the perovskite's properties. We present a method for the rational design of AMO3−δ perovskite solid solutions with two different species on the Msite in order to tune their redox behavior. To account for the different ionic radii of different transition metal species M, two distinct ions are used in a solid solution on the A site, allowing tolerance-factor adjusted materials design. Using this methodology, we can create stable perovskites over a large range of different compositions. Leveraging the infrastructure of Materials Project, we calculate redox enthalpies for the reduction of over 240 of these 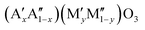 perovskites to their brownmillerite phases based on density functional theory (DFT). We compare this data to experimentally measured data on thermodynamics of 24 of these materials to verify our theoretical framework. An empirical model is formulated for predicting the enthalpy and entropy changes as a function of the perovskites non-stoichiometry δ, which can be used to simulate the equilibrium composition as a function of temperature and oxygen partial pressure and to create a perovskite search engine based on an energetic analysis of the redox cycles. The data has been added as a contribution to MPContribs, which now includes publicly available user-controlled interactive graphs based on our theoretical and experimental data
perovskites to their brownmillerite phases based on density functional theory (DFT). We compare this data to experimentally measured data on thermodynamics of 24 of these materials to verify our theoretical framework. An empirical model is formulated for predicting the enthalpy and entropy changes as a function of the perovskites non-stoichiometry δ, which can be used to simulate the equilibrium composition as a function of temperature and oxygen partial pressure and to create a perovskite search engine based on an energetic analysis of the redox cycles. The data has been added as a contribution to MPContribs, which now includes publicly available user-controlled interactive graphs based on our theoretical and experimental data